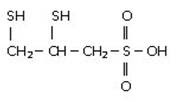
- DMPS is short for 2,3-dimercapto-1-propanesulfonic acid (pronounced dye-mer-cap-toe-pro-pain-sull-fon-ic acid).
- The molecular formula is C3H8S3O3.
- DMPS is called a chelating agent (polydentate ligand), which is a substance that binds to metal.
- DMPS specifically binds to mercury, lead and arsenic, and forms stable complexes with these metals, allowing them to be eliminated from the body.
- DMPS is water soluble, and is over 50% rapidly absorbed through oral administration.
- The sodium salt of DMPS (Unithiol) is effective in lowering the body burden of mercury and also in decreasing the urinary mercury concentration. Therefore, it can be used as an alternative medicine to reduce heavy metal burden in chronic patients

Is DMPS a natural substance?
DMPS is created through a series of complex chemical reactions which is not found in nature. It was first developed in the 1950s in Russia for the treatment of mercury and lead poisoning and has been sold under the brand names Dimaval and Unithiol in Germany and Russia, respectively.
Does DMPS Safe?- DMPS is a substance with very low toxicity systemically and is generally well tolerated, even with long-term use.
- Studies have been conducted on the lowest SINGLE oral dose of DMPS required to cause negative changes to blood chemistry in animals.
- Extensive animal testing in the 1950s with DMPS showed no toxicity to the immune system, heart, liver, blood orkidneys. No significant effects on fetal development were noted when fed to pregnant mice.
- We can find no reports of any human deaths from oral DMPS usage. Even with this safety history, we do NOT provide the MercOut Program to anyone who is pregnant or lactating, or believes there is any possibility she may be pregnant.
Does DMPS Have Any Side Effects?
- DMPS can weakly bond to essential minerals in the body such as copper and zinc. In addition to the possible increased elimination of essential trace elements (especially zinc), allergic reactions in the form of skin reaction are seen in between 5-10% of individuals taking DMPS.
- These reactions are much milder than with the intravenous route of administration. Most of the reported adverse reactions to DMPS occurred on long-term therapy.
- Symptoms were generally mild and included itching, nausea, dizziness, fever, weakness, skin reactions (e.g., rash, urticaria), mucous membrane reactions, raised body temperature, or shivering and fever. Allergic reactions generally subside within 3 to 5 days of stopping DMPS treatment.
- Some people may notice an odor of rotten eggs coming from body excretions (urine, sweat) while they are taking DMPS. This is due to the sulfur nature of the DMPS chemical.

In chemistry, a ligand is either an atom, ion, or molecule that bonds to a central metal, generally involving formal donation of one or more of its electrons. The metal-ligand bonding ranges from covalent to more ionic. Furthermore, the metal-ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known involving Lewis acidic.
Ligands are classified in many ways: their charge, size (bulk), the identity of the coordinating atom(s), and the number of electrons donated to the metal (denticity or hapticity). The size of a ligand is indicated by its cone angle.
Type of Ligand
i)Monodentate ligand
- are ligands which coordinates through only one atom. Some examples are H2O, NH3, P(CH3)3, etc.
ii)Polydentate ligand
- are ligands which coordinate through two or more donor atoms.
- also referred to as chelating ligands when the donor atoms are bonded to the same metal centre.
- Polydentate ligand can be divided into 3 :
1. Bidentate ligand
- have two lone pairs, both can bond to the central metal ion.
- examples are 1,2-diaminoethane
2. Tetradentate ligand
- Has four lone pairs, all of which can bond to the central metal ion.
- Examples are vitamin B-12.
- Vitamin B-12 is required in the diet of all higher animals which is only synthesized by certain bacteria and molds.
3. Hexadentate ligand
- Has six lone pairs of electrons - all of which can form coordinate bonds with the same metal ion.
- The best example is EDTA.

1. In coordination compounds, central metal ions exhibit both primary and secondary valences
- the primary valency is ionizable
- the secondary valency is not ionizable
- the secondary valency corresponds to coordination number (the central metal ion and ligands are not ionizable)
2. Every metal atom has a fixed number of secondary valencies (coordination number(s))
3. The metal atom tends to satisfy both its primary valency as well as its secondary valency
- primary valency is satisfied by negative ions (metal ion has a positive charge)
- secondary valency (coordination number) is satisfied either by negative ions or by neutral molecules
- in certain cases, a negative ion may satisfy both types of valencies
4. The coordination number (secondary valencies) are always directed towards the fixed positions in space and this leads to definite geometry of the coordination compound
DMPS related to Werner’s Theory
- In this case, the primary valency is the central metal ions and the secondary valency is the DMPS
- For mercury, the primary valency (oxidation number) is +2 and the secondary valency corresponds to coordination number, which is 4.

- Mercury is toxic to the body cell
- Therefore, if mercury is found in human body, it has to be removed
- Doctors will administer the sodium salt of DMPS (Unithiol) as medicine to the patients with mercury poisoning
- In the body, the sodium salt of DMPS complex will dissociate into sodium and DMPS
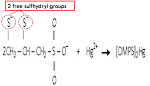
- DMPS will form a coordination compound with mercury = [DMPS]2Hg
- This is because DMPS is a chelating agent which contains 2 SH groups (di-thiol)
- Hg ion has great affinity for SH groups of endogenous biomolecules, which may contribute to its toxicity
- Since DMPS has 2 SH groups, injection of the DMPS can bind tightly to Hg
- The product of this compound ([DMPS]2Hg) will be eliminate from the body through the excretion system

![Structural formula of [DMPS]2Hg complex](http://2.bp.blogspot.com/_9FBQIUmSu30/SZmHQ8BfSxI/AAAAAAAAAAc/vtB4qCwcp6Y/S265/Picture3.jpg)